Products
Manual Titrator
Automatic Titrator
Vialab has selected a test set for manually determining the acid number.
It is suitable for occasional analysis.
SINGLE EQUIVALENCE POINT
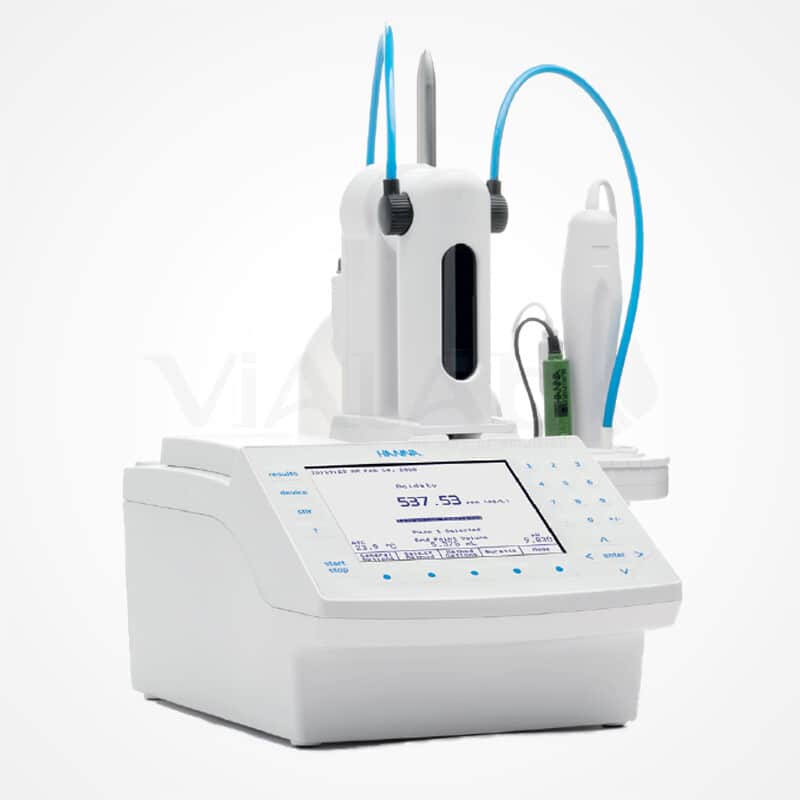
Accurate, fast and reliable, the Vialab Automatic Titrator increases the accuracy and reliability of results while reducing the duration of the test. By integrating its methods (dosage of the titrating solution and determination of the acid number of bitumens), Vialab offers a high-performance device, perfectly suited to your needs.
DOUBLE EQUIVALENCE POINT
Accurate, fast and reliable, the Vialab Automatic Titrator increases the accuracy and reliability of results while reducing the duration of the test. By integrating its methods (dosage of the titrating solution and determination of the acid number of bitumens), Vialab offers a high-performance device, perfectly suited to your needs.
Accessories
PROBE pH/mV | SPECIAL EMULSION
Specifications
- Reference Single, Ag / AgCl
- Junction Ceramic reinforced triple
- Electrolyte KCl 3.5M + AgCl
- Max pressure 0.1 bar
- pH range: 0 to 12 ° T: -5 to 100 ° C
- Tapered Tip (12 x 12 mm)
- Temperature sensor: No
- Amplifier: No
- Body Material: Glass
- Coaxial cable **, 1 m (3.3 ‘)
F188.120.0055
BURETTE 10 ML | AUTOMATIC TITRATOR
Specifications
Burette 10ml for the determination of the titrating solution with benzoic acid.
- Seryngue 10ml in frosted glass and plunger
- Suction and distribution tubing
- Complies with the XP CEN / TS 17482 standard which recommends burettes with a volume of 5 to 20ml
F188.120.4821
BROWN GLASS BOTTLE | 1000 ML
Specifications
- Useful volume 1000 ml
- Borosilicate glass Brown for protection against light
- Blue safety ring and cap PP
- Screw neck
- Pack of 10
F039.305.1104
BEAKER | HIGH FORM | 150 ML
Specifications
- High shape with spout
- Borosilicate Glass
- White graduation
- Pack of 10
F039.310.6015
MOHR BURETTE | CLASS A | 25/0.05 ML
Specifications
- Integrated funnel
- PTFE stopcock
- ISO 385
- Blue graduation
- Class A
F039.327.7015
DOUBLE CLAMP FOR BURETTE
Specifications
- Self adjusting clamp for 2 burettes, with central bosshead screw.
- Made of Chrome plated metal with PVC coated jaws.
- Enables complete visibility of the burette scalewhile being held.
F039.205.0181
MINI MAGNETIC STIRRER
Specifications
- Robust
- PET stirring surface
- Excellent chemical resistance
- Stirring speed from 300 to 2000 rpm
- Volume up to 1.5L
- Ø of the plate: 120 mm
- Supplied with rod and electrode clamp
- Power supply: 220/230 VAC / 50 Hz
F039.115.5068
TRIPOD BASE | Ø 280 mm | M10
Specifications
- Painted cast steel base
- Star tripods (3 supports)
- Rubber pad under the base
F039.205.6514
STAINLESS STEEL ROD | 600 mm | M10
Specifications
Consult us
ISO GLASS BOTTLE | 1000 ML | CAP
Specifications
- Useful volume 1000 ml
- Borosilicate glass
- Blue safety ring and cap
- Screw neck
- Pack of 10
F039.305.1100
Consumables
ELECTRODE CLEANING SOLUTION
Specifications
- Bottle of 500 ml
- Cleaning solution for pH electrode
F188.120.7061